Background:
In multiple myeloma, induction therapy (IT) before hematopoietic progenitor cells (HPC) collection reduces the tumor burden, improves the quality of the collection, and diminishes end-organ damage. The data concerning the impact of the response to IT on progression-free survival (PFS) after autologous stem cell transplant (ASCT) remains limited. IFM 2005-01 reported better PFS in patients who achieved at least a very good partial response (VGPR) after IT (PFS post-ASCT 41 vs. 31 months, p= 0.01). Also, studies have consistently shown that minimal residual disease (MRD) negativity impacted PFS/ overall survival (OS).
Currently, rates of CR and MRD-negativity post-ASCT are sub-optimal. Given the clinical activity and the safety profile of daratumumab (Dara), evaluation of this novel agent pre- and post-ASCT is warranted, as it may improve the post-ASCT ≥ CR and MRD-negativity rates.
Study Design and Methods:
This study is a single-arm, two-stage phase II trial to estimate the CR rate post-ASCT in newly diagnosed MM (NDMM). Transplant eligible (TE) NDMM subjects who did not achieve at least a VGPR post initial IT are eligible for the trial.
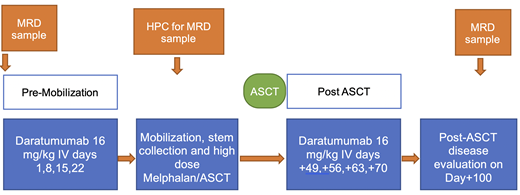
Enrollment will be planned post-induction and before HPC mobilization. Subjects will receive four weekly doses of Dara prior to HPC mobilization, then another four weekly doses of Dara after HPC engraftment post-ASCT.
The primary objective is to evaluate the ≥ CR rate post-ASCT in NDMM subjects who did not achieve at least a VGPR post-induction.
The secondary objectives are to estimate: ≥VGPR rate, time to first response (TTFR), time to best response (TTBR), duration of response (DoR), PFS, time to progression (TTP), time to next treatment (TTNT), and OS. Also, the study will evaluate toxicities related to Dara and ASCT outcomes (HPC collection and engraftment parameters).
The exploratory objectives are to explore:
MRD rates of bone marrow and HPC product using Euro-flow criteria and DNA-PCR.
The correlation between systemic immune profiling and the clinical response using cytokine profiling by multiplex protein assay and Blood immunotyping [including NK, NK-T, and T cell subsets distribution and activation analyses by flow cytometry.
The correlation between circulating T cell receptor (TCR) repertoire immuno-sequencing by next-generation sequencing (NGS) with clinical response parameters.
The changes in BM and BM plasma cells' biology before and after treatment by Whole Exome Sequencing, and
The correlation between PET/CT responses and endpoint.
The main eligibility criteria are age ≥ 18 years, ECOG PS 0-2, measurable disease at the time of diagnosis, < VGPR per IMWG 2016 criteria following a 3-drug IT regimen for TE-NDMM. However, patients must have achieved at least a minimal response, and treatment plan includes ASCT post-induction.
Statistical methods:
Post-ASCT ≥CR response is the primary endpoint for this study. For NDMM, standard therapy strategies provide ≥CR rates of approximately 50%. For this population of subjects treated with Dara-based in vivo purging, the aim is to achieve a post-ASCT ≥ CR rate of 70%. A minimax 2-stage design will be used to test the hypothesis that the ≥ CR rate post-ASCT is less than or equal to 50%. Twenty-three subjects will be enrolled in the first stage, and if at least 12 of the 23 subjects have ≥CR after ASCT, an additional 16 subjects will be enrolled (total of 39 subjects). If at least 24 of 39 subjects have ≥CR after ASCT, the null hypothesis will be rejected. Based on a one-sided, α = 0.10 significance level, this sample size will provide 90% power to reject the null, assuming the true ≥CR rate post-ASCT is 70%.
The treatment schedule:
Pre-mobilization Dara on days 1,8,15,22. Four weeks after the Day 22 dose of Dara, all subjects will undergo HPC mobilization with the hematopoietic growth factor G-CSF with or without the chemokine receptor type 4 (CXCR4) antagonist. Post-ASCT and after engraftment is complete, Dara will be restarted days +49, +56, +63, +70.
ClinicalTrials.gov Identifier: NCT04230031
Atrash:Amgen, GSK, Karyopharm.: Research Funding; Takeda, Amgen, Karyopharm, BMS, Sanofi, Cellactar, Janssen and Celgene: Honoraria; BMS, Jansen oncology, Sanofi: Speakers Bureau. Paul:Regeneron: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Other: Stock Ownership (prior employee). Symanowski:Casgen: Consultancy; Eli Lilly: Consultancy; Immatics: Consultancy; Novartis: Consultancy. Bhutani:Janssen: Other: Clinical Trial Funding to Institute; BMS: Other: Clinical trial funding to institute, Speakers Bureau; Amgen: Speakers Bureau; Prothena: Other: Clinical Trial Funding to Institute; Sanofi Genzyme: Consultancy; Takeda: Other: Clinical trial funding to institute, Speakers Bureau; MedImmune: Other: Clinical Trial Funding to Institute. Voorhees:Bristol-Myers Squibb: Other: Personal fees; Celgene: Other: Personal fees; Janssen: Other: Personal fees; Novartis: Other: Personal fees; Oncopeptides: Other: Personal fees; TeneoBio: Other: Personal fees; Levine Cancer Institute, Atrium Health: Current Employment; Adaptive Biotechnologies: Other: Personal fees. Usmani:Merck: Consultancy, Research Funding; Incyte: Research Funding; Pharmacyclics: Research Funding; Array Biopharma: Research Funding; GSK: Consultancy, Research Funding; Celgene: Other; Amgen: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; BMS, Celgene: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Abbvie: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; Janssen: Consultancy, Honoraria, Other: Speaking Fees, Research Funding; SkylineDX: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal